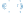0 of 9 questions completed
Questions:
This test is comprised of 9 items, which must be completed within 12 minutes.
Each test item is a question or incomplete statement followed by suggested answers or completions. Read the item, decide which choice is best, and select the answer with the mouse. A navigation bar is provided, enabling you to skip between questions easily and mark questions for review. |
You have already completed the quiz before. Hence you can not start it again.
Exam is loading ...
You must sign in or sign up to start the exam.
You have to finish following exam, to start this exam:
0 of 9 questions answered correctly
Your time:
Time has elapsed
You answered 0 of 0 (0) questions correct
| Average score |
|
| Your score |
|
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 118
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 119
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 120
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 121
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 122
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Learn from your mistakes so you don’t repeat them on the next exam. Keep your head up and keep on fighting! Pretty soon you’ll see your scores go up from here!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 123
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting! What is one thing you’ll do different on the next test?
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 124
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 125
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 126
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 127
Great job! You are really getting to where you need to be. Keep it going! Sit down and reflect and learn from what prevented you from getting a 128. Keep on working on it and you’ll soon see your score hit 127+. Take your time in understanding your mistakes and in carefully reviewing these solutions and understanding the intricacies of each question. Your goal should be to beat your 127 on the next test!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 128
Good going there! You hit 128! Take your time in understanding your mistakes and in carefully reviewing these solutions and understanding the intricacies of each question. Your goal should be to beat your 128 on the next test!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 129
Impressive! You’re a super star!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 130
Awesome job! Learn from your mistakes and strategize on how you’ll beat your 130!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 131
Impressive! You’re a super star!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
Estimated MCAT Score for the “Chemical and Physical Foundations of Living Systems” section: 132
You are a rockstar! We tip our hats to you!
Also, don’t focus on your estimated score, they mean essentially nothing at the start. Rarely does anyone start these exams and score well immediately, if that was the case then they wouldn’t even need to practice! These are ‘practice’ tests, meaning you’re practicing to improve your skills. If you continue to work hard and study, read and understand the solutions, practice questions daily, and give it your best effort, we promise your score will improve. Review and learn for now, and the scores will come. Upgrade your membership to get instant access to all of our MCAT Material to help you ace your exam.
1) Water has several unique properties that allow it to serve as the primary solvent in biological systems. The intrinsic property of a fluid that allows it to resist external forces is known as the
Answer: C
Foundational Concept: 4
Content Category: 4B
Skill: 1
The intermolecular forces within a liquid influence its behavior. The surface tension is the attraction of the surface molecules that causes the liquid’s surface to contract and become more spherical. The surface tension of a liquid is important because it enables it to resist external forces. For example, some objects can float on the surface of a liquid without “breaking” the surface. The correct response to the question is (C). Cohesion (A) is the property of water that allows the molecules to cohere or stick together. This allows the molecules to work together, creating unique properties such as the ability of water to expand when it freezes. Viscosity (D) is a liquid’s resistance to flow, and miscibility (B) is the ability of a liquid to dissolve in a solvent such as water.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 11.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: C
Foundational Concept: 4
Content Category: 4B
Skill: 1
The intermolecular forces within a liquid influence its behavior. The surface tension is the attraction of the surface molecules that causes the liquid’s surface to contract and become more spherical. The surface tension of a liquid is important because it enables it to resist external forces. For example, some objects can float on the surface of a liquid without “breaking” the surface. The correct response to the question is (C). Cohesion (A) is the property of water that allows the molecules to cohere or stick together. This allows the molecules to work together, creating unique properties such as the ability of water to expand when it freezes. Viscosity (D) is a liquid’s resistance to flow, and miscibility (B) is the ability of a liquid to dissolve in a solvent such as water.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 11.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
2) What is the IUPAC name for the compound below?
The compound contains a carbonyl group (C=O) in ketone, an ether (C-O-C), and a cyclic alkane. In naming the compound, the ketone takes precedence. The suffix for the name will end in –one.
In general the precedence for the suffix is as follows:
Carboxylic acid > carboxylic acid derivatives (esters, acyl halides, amides, imides, amidines) aldehyde > ketone > alcohol > amino > sulfhydryl
To name the compound:
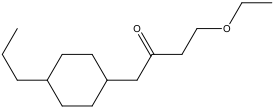
Number the chain that contains the ketone:
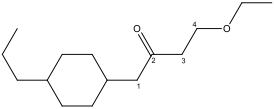
B) Treat the remaining groups as attachments to the ketone chain:
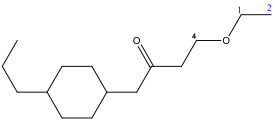
4-ethoxy: 2 carbons forming one arm of an ether.
For the cycloalkene: When numbering the carbons of a cycloalkane, start with a substituted carbon so that the substituted carbons have the lowest numbers (sum):

4-propylycyclohexane
C) Combine the parts to write the complete name:

The correct response to the question is (C). The compound is a ketone-ether and not an ester (A), (B), and (D).
[crack_lightbox name=”videos/chem/Chem Test 1 Q 12.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
The compound contains a carbonyl group (C=O) in ketone, an ether (C-O-C), and a cyclic alkane. In naming the compound, the ketone takes precedence. The suffix for the name will end in –one.
In general the precedence for the suffix is as follows:
Carboxylic acid > carboxylic acid derivatives (esters, acyl halides, amides, imides, amidines) aldehyde > ketone > alcohol > amino > sulfhydryl
To name the compound:
Number the chain that contains the ketone:

B) Treat the remaining groups as attachments to the ketone chain:

4-ethoxy: 2 carbons forming one arm of an ether.
For the cycloalkene: When numbering the carbons of a cycloalkane, start with a substituted carbon so that the substituted carbons have the lowest numbers (sum):

4-propylycyclohexane
C) Combine the parts to write the complete name:

The correct response to the question is (C). The compound is a ketone-ether and not an ester (A), (B), and (D).
[crack_lightbox name=”videos/chem/Chem Test 1 Q 12.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
3) During glycolysis in the cell, the oxidation of glyceraldehyde 3-phosphate (PGAL) occurs by the removal of electrons accompanied by hydrogens, which are picked up by NAD+ to form NADH. At the same time, ADP is converted to ATP by the transfer of the phosphate group from PGAL. This is an example of:
Answer: B
Foundational Concept: 5
Content Category: 5E
Skill: 2
This step in glycolysis generates two molecules of ATP and two molecules of NADH. NAD+ molecules accept electrons from PGAL, and form NADH. NADH delivers the hydrogen atoms to the electron transport chain if possible. The simultaneous transfer of the phosphate group from PGAL to ADP to form ATP is referred to as substrate level phosphorylation (B). Therefore, the correct answer is (B). In substrate level phosphorylation (B), ATP is created by the direct transfer of a phosphate group from a donor molecule in the metabolic pathway. In oxidative phosphorylation (A), electrons are transferred from electron donors to electron acceptors in a series of redox reactions. The energy released by these redox reactions is used to form ATP. This is observed in the electron transport chain. Although these reactions are facilitated by enzymes, the processes themselves are not examples of catalysis (C), and choices (C) and (D) are both incorrect.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 13.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: B
Foundational Concept: 5
Content Category: 5E
Skill: 2
This step in glycolysis generates two molecules of ATP and two molecules of NADH. NAD+ molecules accept electrons from PGAL, and form NADH. NADH delivers the hydrogen atoms to the electron transport chain if possible. The simultaneous transfer of the phosphate group from PGAL to ADP to form ATP is referred to as substrate level phosphorylation (B). Therefore, the correct answer is (B). In substrate level phosphorylation (B), ATP is created by the direct transfer of a phosphate group from a donor molecule in the metabolic pathway. In oxidative phosphorylation (A), electrons are transferred from electron donors to electron acceptors in a series of redox reactions. The energy released by these redox reactions is used to form ATP. This is observed in the electron transport chain. Although these reactions are facilitated by enzymes, the processes themselves are not examples of catalysis (C), and choices (C) and (D) are both incorrect.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 13.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
4) Many imaging techniques and instruments are vital to the proper diagnosis of many medical conditions. When a real object is viewed with a diverging lens, the image appears
Answer: C
Foundational Concept: 4
Content Category: 4D
Skill: 1
Images are characterized in the following ways: (1.) virtual or real, (2.) upright or inverted, (3.) reduced, enlarged, same size. The two types of lenses are shown below:
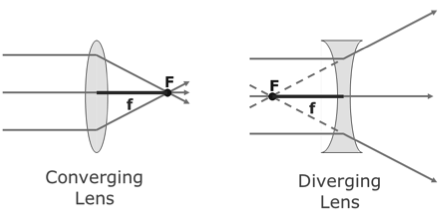
Real images arise when objects are placed outside the focal length of a converging lens or outside the focal length of a converging mirror. Virtual images are made by diverging lenses or by placing an object inside the focal length of a converging lens. Diverging lenses form reduced, erect, virtual images.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 14.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: C
Foundational Concept: 4
Content Category: 4D
Skill: 1
Images are characterized in the following ways: (1.) virtual or real, (2.) upright or inverted, (3.) reduced, enlarged, same size. The two types of lenses are shown below:

Real images arise when objects are placed outside the focal length of a converging lens or outside the focal length of a converging mirror. Virtual images are made by diverging lenses or by placing an object inside the focal length of a converging lens. Diverging lenses form reduced, erect, virtual images.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 14.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
5) A student wants to perform an experiment that requires the results of a titration. Which of the following is not needed to complete an acid-base titration?
Answer: C
Foundational Concept: 5
Content Category: 5A
Skill: 1
A titration is a standard technique used to determine the concentration of an unknown solution. Acid base titrations are frequently completed to identify an unknown acid or base in a solution, by neutralizing the solution. The key pieces of equipment in a titration are a burette (A), pipette, pH indicator (B), flask, titrant, and analyte (D). The burette is used to dispense specific amounts of the titrant, or known compound. The unknown compound, or the analyte (D), is transferred with a pipette into the flask below the burette. The pH indicator (B) is used to signal the end of the titration. When the acid or base has been neutralized completely, the pH indicator changes color permanently. A buffer (C) is a solution in which the pH changes very little when a small amount of acid or base is added to it. Buffers are not used in acid base titrations, and the correct response is (C).
[crack_lightbox name=”videos/chem/Chem Test 1 Q 15.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: C
Foundational Concept: 5
Content Category: 5A
Skill: 1
A titration is a standard technique used to determine the concentration of an unknown solution. Acid base titrations are frequently completed to identify an unknown acid or base in a solution, by neutralizing the solution. The key pieces of equipment in a titration are a burette (A), pipette, pH indicator (B), flask, titrant, and analyte (D). The burette is used to dispense specific amounts of the titrant, or known compound. The unknown compound, or the analyte (D), is transferred with a pipette into the flask below the burette. The pH indicator (B) is used to signal the end of the titration. When the acid or base has been neutralized completely, the pH indicator changes color permanently. A buffer (C) is a solution in which the pH changes very little when a small amount of acid or base is added to it. Buffers are not used in acid base titrations, and the correct response is (C).
[crack_lightbox name=”videos/chem/Chem Test 1 Q 15.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
6) Materials that are diamagnetic can induce a magnetic field in opposition to another magnetic field that is externally applied. Which of the following is a diamagnetic atom?
Answer: D
Foundational Concept: 4
Content Category: 4E
Skill: 1
Magnetism is the ability of a material to respond to a magnetic field. Atoms display magnetism, and can be classified as diamagnetic or paramagnetic. The distinction is based on the electron configuration of the atom. The electron configuration is the arrangement of the electrons in an element’s energy shells. The electron configuration of an atom is typically written for the ground state, or lowest energy state, of an atom. Electrons are found in pairs inside of the orbitals in an atom. The electrons are spin-paired; each goes in the opposite direction of other. In the diamagnetic atoms, each orbital in a shell of the atom is completely filled. As a result, diamagnetic atoms are not affected by magnetic fields; rather they are repelled. If an atom only has spin paired electrons, it is diamagnetic. Paramagnetic atoms have unfilled orbitals in their shells, and they are sensitive to magnetic fields in their environments. Therefore, paramagnetic atoms are attracted to a magnetic field.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 16.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: D
Foundational Concept: 4
Content Category: 4E
Skill: 1
Magnetism is the ability of a material to respond to a magnetic field. Atoms display magnetism, and can be classified as diamagnetic or paramagnetic. The distinction is based on the electron configuration of the atom. The electron configuration is the arrangement of the electrons in an element’s energy shells. The electron configuration of an atom is typically written for the ground state, or lowest energy state, of an atom. Electrons are found in pairs inside of the orbitals in an atom. The electrons are spin-paired; each goes in the opposite direction of other. In the diamagnetic atoms, each orbital in a shell of the atom is completely filled. As a result, diamagnetic atoms are not affected by magnetic fields; rather they are repelled. If an atom only has spin paired electrons, it is diamagnetic. Paramagnetic atoms have unfilled orbitals in their shells, and they are sensitive to magnetic fields in their environments. Therefore, paramagnetic atoms are attracted to a magnetic field.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 16.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Passage #1:
[textwrap]Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.
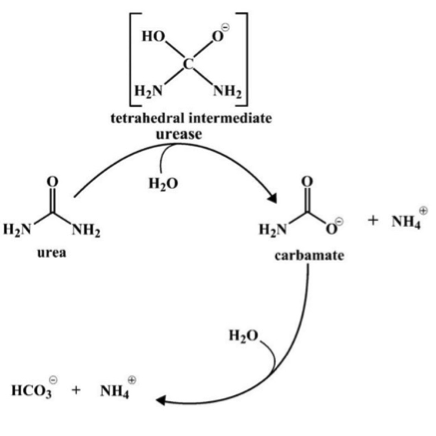
Reaction 1
Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.
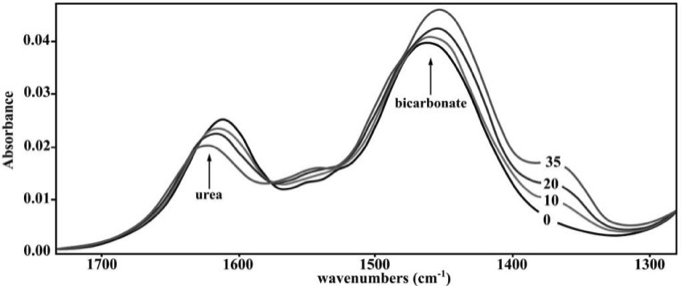
Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
[/textwrap]
7) Which of the following represents a balanced equation for urea hydrolysis?
Answer: C
Foundational Concept: 5
Content Category: 5E
Skill: 1
The balanced reaction is shown below:
[crack_lightbox name=”videos/chem/Chem Test 1 Q 22.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
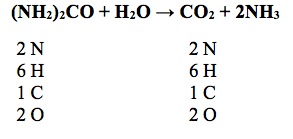
Answer: C
Foundational Concept: 5
Content Category: 5E
Skill: 1
The balanced reaction is shown below:
[crack_lightbox name=”videos/chem/Chem Test 1 Q 22.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
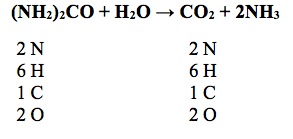
Passage #1:
[textwrap]Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Reaction 1
Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
[/textwrap]
8) The researchers were able to use FTIR spectroscopy to analyze urease activity because
Answer: B
Foundational Concept: 4
Content Category: 4D
Skill: 4
The technique allows simultaneous observation of the disappearance of the substrate and the appearance of the product. The spectra for the both the substrate (urea) and product (bicarbonate) are present in Figure 1.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 23.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: B
Foundational Concept: 4
Content Category: 4D
Skill: 4
The technique allows simultaneous observation of the disappearance of the substrate and the appearance of the product. The spectra for the both the substrate (urea) and product (bicarbonate) are present in Figure 1.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 23.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Passage #1:
[textwrap]Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Reaction 1
Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
[/textwrap]
9) The protonation of the ammonia in the urea hydrolysis reaction shown in Reaction 1, which was carried out in water, leads to which of the following?
Answer: A
Foundational Concept: 5
Content Category: 5A
Skill: 4
As shown by Reaction 1, in water the carbamate spontaneously converts into the second ammonia molecule and carbonic acid. Then, the addition of a proton to a base such as ammonia (NH3) increases the pH of the solution, making it more alkaline or basic.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 24.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]
Answer: A
Foundational Concept: 5
Content Category: 5A
Skill: 4
As shown by Reaction 1, in water the carbamate spontaneously converts into the second ammonia molecule and carbonic acid. Then, the addition of a proton to a base such as ammonia (NH3) increases the pH of the solution, making it more alkaline or basic.
[crack_lightbox name=”videos/chem/Chem Test 1 Q 24.mp4″ bucket=”mcatcracker” width=”” height=”” text=”Click here to open the video explanation” autoplay=”false”]